Pipeline
The H&B JISHI’s portfolio of phase II/III product candidates comprises UC-MSC for steroid-refractory acute graft versus host disease (acute GVHD), for the treatment of moderate to severe acute-on-chronic liver failure (ACLF). The phase I/II product candidates include UC-MSC for refractory immune thrombocytopenia (ITP), spinal cord injury (SCI), stroke, as well as acute respiratory distress syndrome (ARDS). P-MSC hydrogel for diabetic foot ulcer and radiation skin injury. CD106+MSC for critical limb ischemia.
|
Product name |
Indication (trial stage) |
|
UC-MSC Injection |
SR-GVHD (phase 2/3) |
|
Acute-on-chronic liver failure (phase 2/3) |
|
|
Acute Respiratory Distress Symdrome (Phase1/2) |
|
|
Spinal cord injury (Phase 1/2) |
|
|
Refractory immune thrombocytopenia (Phase 1/2) |
|
|
VCAM-1+MSC injection |
Critical limb ischemia (Phase 1/2) |
|
Stroke (Phase 1/2) |
|
|
Placenta MSC hydrogel |
Diabetic foot ulcer (Phase 1/2) |
|
Radiation skin injury (Phase 1/2) |
NECCP
H&B JISHI has established the National Engineering Center of Cell Products in Tianjin. This center is at the forefront of research and development in China's emerging field of cell-based drugs and aims to become a pioneering enterprise leading international advancements in cellular technologies.




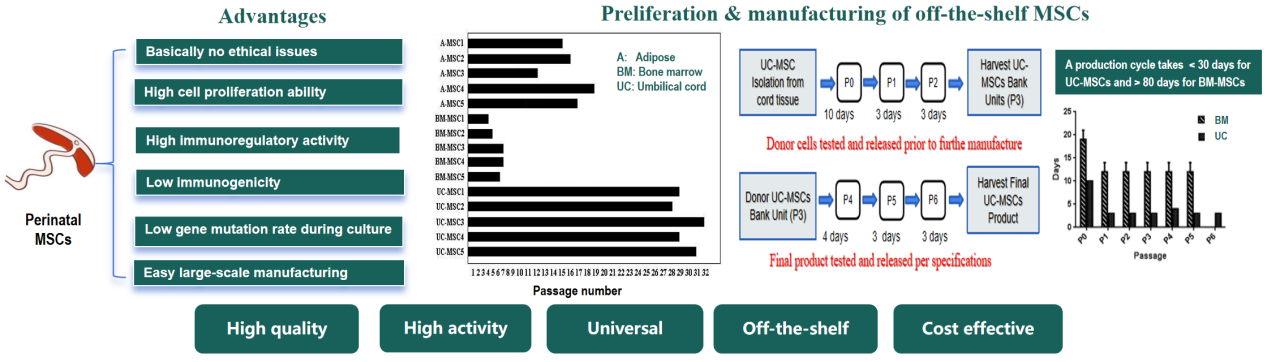
Advantages of perinatal MSCs
MSCs derived from perinatal tissues or fluids, including placenta, umbilical cord, cord blood and amniotic fluid. These stem cells are acquired after birth, they are not immortal but have a high level of cell division, and are multipotent. The perinatal MSCss are generally not distinguished from adult MSCs but with several advantages in comparison with MSCs derived from adult tissues including bone marrow and adipose (see the figure below).